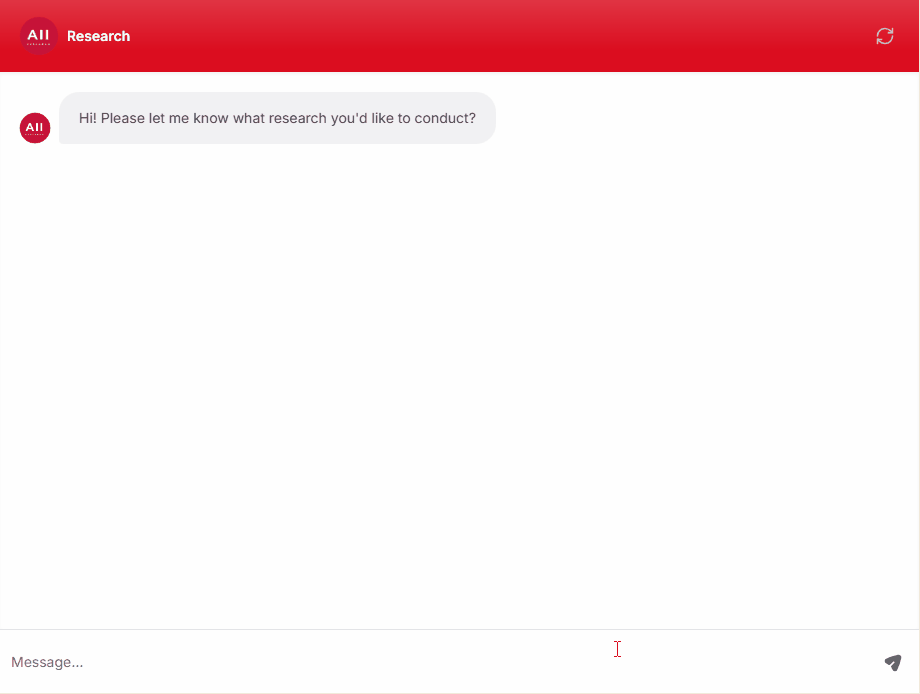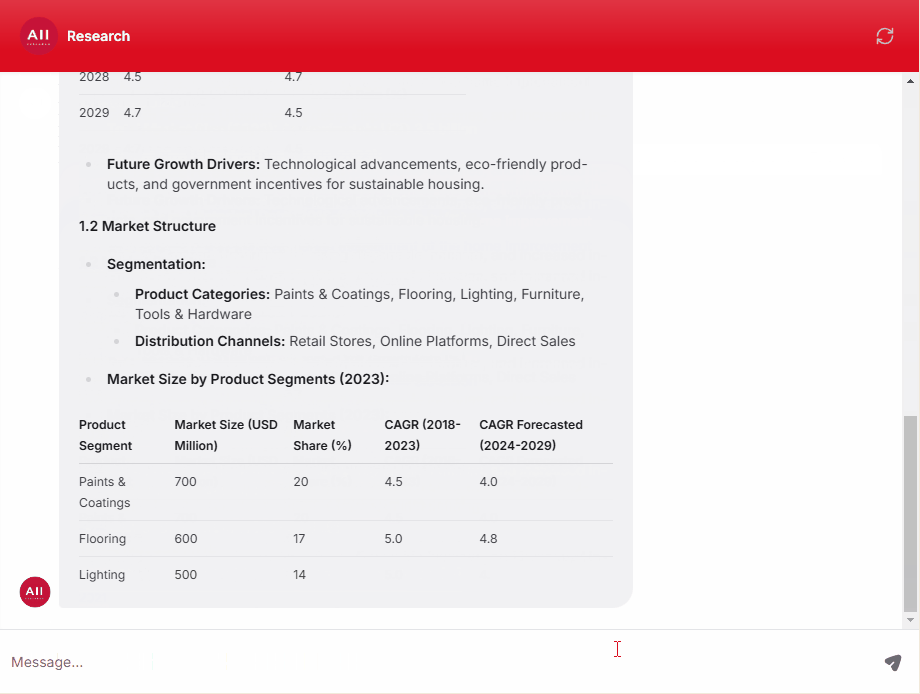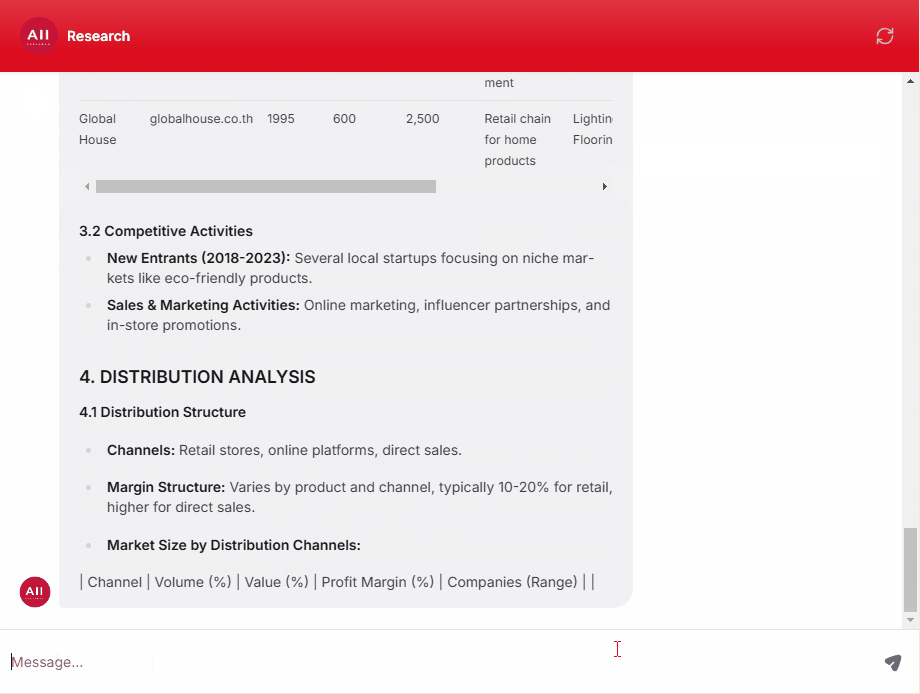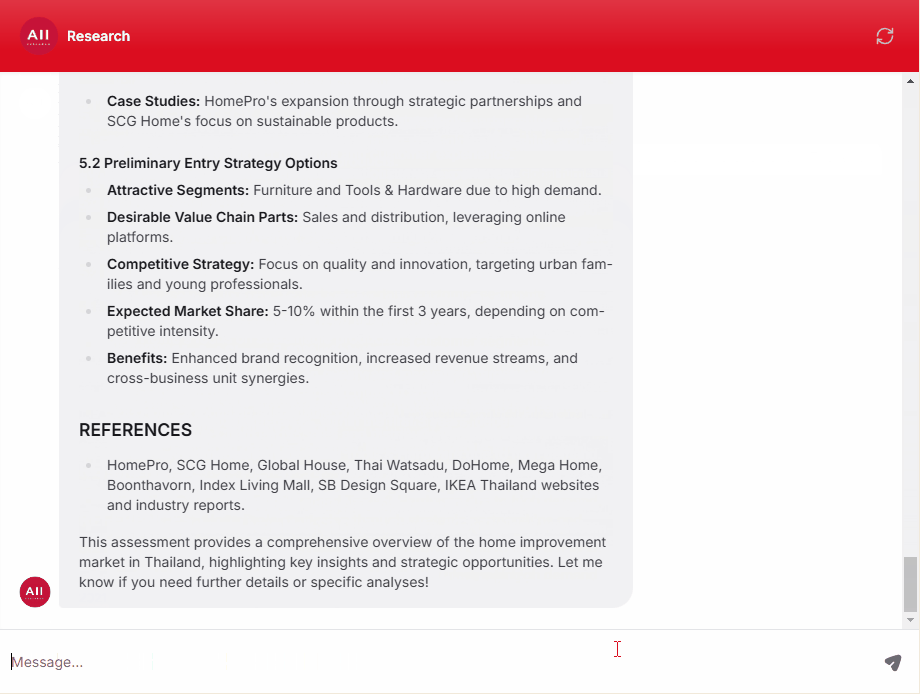
Clinical, Medical, and Regulatory
Departments responsible for ensuring that the product meets all clinical and regulatory requirements and managing medical communication.
Implications
The aspects of pharmaceutical and healthcare development that involve clinical trials, medical evaluations, and compliance with regulatory requirements, essential for bringing new drugs and medical devices to market safely and effectively.
Example
Example: A pharmaceutical company�s Clinical, Medical, and Regulatory team oversees the design and execution of clinical trials, ensures compliance with FDA regulations, and manages medical affairs to support the approval and commercialization of a new drug.
Related Terms
Different from R&D, which focuses on the discovery and development of new products, Clinical, Medical, and Regulatory functions are specifically concerned with ensuring that products meet safety, efficacy, and legal standards.